HAUPTMENÜ
AWARDS
Forschergeist gefragt: 14. Novartis Oppenheim-Förderpreis für MS-Forschung ausgelobt
FernstudiumCheck Award: Deutschlands beliebteste Fernhochschule bleibt die SRH Fernhochschule
Vergabe der Wissenschaftspreise der Deutschen Hochdruckliga und der Deutschen Hypertoniestiftung
Den Patientenwillen auf der Intensivstation im Blick: Dr. Anna-Henrikje Seidlein…
Wissenschaft mit Auszeichnung: Herausragende Nachwuchsforscher auf der Jahrestagung der Deutschen…
VERANSTALTUNGEN
Wichtigster Kongress für Lungen- und Beatmungsmedizin ist erfolgreich gestartet
Virtuelle DGHO-Frühjahrstagungsreihe am 22.03. / 29.03. / 26.04.2023: Herausforderungen in…
Pneumologie-Kongress vom 29. März bis 1. April im Congress Center…
Die Hot Topics der Hirnforschung auf dem DGKN-Kongress für Klinische…
Deutscher Schmerz- und Palliativtag 2023 startet am 14.3.
DOC-CHECK LOGIN
Eleven new medicines, including one orphan, recommended for approval
Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 20-23 April 2015
London (April 24, 2015) – Eleven new medicines were recommended for approval at the April 2015 meeting of the Committee for Medicinal Products for Human Use (CHMP).
The CHMP recommended granting a marketing authorisation for Opdivo (nivolumab), for the treatment of adults with advanced (unresectable or metastatic) melanoma. For more information on Opdivo, please see the press release in the grid below.
The Committee recommended granting a marketing authorisation for Hetlioz (tasimelteon) to treat non-24-hour sleep-wake disorder in totally blind adults. Hetlioz was granted orphan designation in 2011. For more information on Hetlioz, please see the press release in the grid below.
Lixiana (edoxaban) received a positive opinion from the Committee for the prevention of stroke and systemic embolism in atrial fibrillation, and the prevention and treatment of venous thromboembolism.
LuMark (Lutetium (177Lu) chloride), a radiopharmaceutical precursor, received a position opinion from the CHMP for the radiolabelling of carrier molecules.
The following seven generic medicines received a positive opinion from the CHMP: Aripiprazole Pharmathen (aripiprazole) for the treatment of schizophrenia and treatment and prevention of manic episodes of bipolar 1 disorder; Aripiprazole Zentiva (aripiprazole) for the treatment of schizophrenia and treatment and prevention of manic episodes in bipolar 1 disorder; Duloxetine Mylan (duloxetine) for the treatment of major depressive disorder, diabetic peripheral neuropathic pain and generalised anxiety disorder; Pregabalin Mylan (pregabalin) for the treatment of epilepsy and generalised anxiety disorder; Pregabalin Mylan Pharma (pregabalin) for the treatment of neuropathic pain, epilepsy and generalised anxiety disorder; Pregabalin Sandoz (pregabalin) for the treatment of epilepsy, neuropathic pain and generalised anxiety disorder and Pregabalin Sandoz GmbH for the treatment of epilepsy and generalised anxiety disorder.
Negative opinion on new medicine
The CHMP adopted a negative opinion for Lympreva (dasiprotimut-T) which was intended for the treatment of patients with follicular non-Hodgkin’s lymphoma.
Six recommendations on extensions of therapeutic indication
The Committee recommended extensions of indication for Esmya, Invega, Levemir, Relistor, Resolor and Tygacil.
CHMP recommends avoiding use of certain hepatitis C medicines with amiodarone
The CHMP confirmed a risk of severe bradycardia or heart block when the hepatitis C medicines Harvoni (sofosbuvir with ledipasvir) or a combination of Sovaldi (sofosbuvir) and Daklinza (daclatasvir) are used in patients who are also taking the antiarrhythmic amiodarone. To manage this risk, the CHMP recommends that in patients taking these medicines, amiodarone should only be used if other antiarrhythmics cannot be given. For more information, please see the public health communication in the grid below.
Withdrawal of application
The application for marketing authorisation for Duloxetine Sandoz (duloxetine) has been withdrawn.
A question-and-answer document on this withdrawal is available in the grid below.
Agenda and minutes
The agenda of the April 2015 meeting is published on EMA’s website. The minutes of the meeting will be published during the week following the May CHMP meeting.
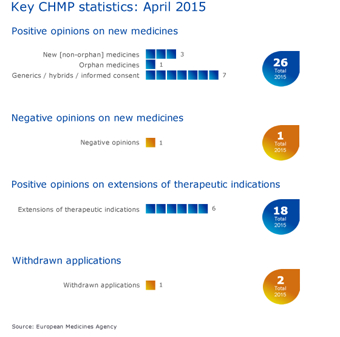
CHMP statistics
Key figures from the April 2015 CHMP meeting are represented in the graphic below.
More information on this, and all other outcomes of the CHMP’s April 2015 meeting, is available in the grid below.

Positive recommendations on new medicines
|
Name of medicine |
Hetlioz |
|
International non-proprietary name (INN) |
tasimelteon |
|
Marketing-authorisation applicant |
Vanda Pharmaceuticals Ltd |
|
Therapeutic indication |
Treatment of non-24-hour sleep-wake disorder (non-24) in totally blind adults |
|
More information |
Summary of opinion for Hetlioz Press release: First EU treatment for rare sleep-wake disorder |
|
Name of medicine |
Lixiana |
|
INN |
edoxaban |
|
Marketing-authorisation applicant |
Daiichi Sankyo Europe GmbH |
|
Therapeutic indication |
Prevention of stroke and systemic embolism in atrial fibrillation |
|
More information |
|
Name of medicine |
Lumark |
|
INN |
Lutetium (177Lu) chloride |
|
Marketing-authorisation applicant |
I.D.B. Radiopharmacy B.V. |
|
Therapeutic indication |
Used only for the radiolabelling of carrier molecules |
|
More information |
|
Name of medicine |
Opdivo |
|
INN |
nivolumab |
|
Marketing-authorisation applicant |
Bristol-Myers Squibb Pharma EEIG |
|
Therapeutic indication |
Treatment of advanced (unresectable or metastatic) melanoma in adults |
|
More information |
Press release: New treatment for advanced melanoma |
Negative recommendation on new medicine
|
Name of medicine |
Lympreva |
|
INN |
dasiprotimut-t |
|
Marketing-authorisation applicant |
Biovest Europe Ltd |
|
Therapeutic indication |
Treatment of non-Hodgkin’s lymphoma (FL) |
|
More information |
Positive recommendations on new generic medicines
|
Name of medicine |
Aripiprazole Pharmathen |
|
INN |
aripiprazole |
|
Marketing-authorisation applicant |
Pharmathen S.A. |
|
Therapeutic indication |
Treatment of schizophrenia and treatment and prevention of manic episodes in bipolar I disorder |
|
More information |
|
Name of medicine |
Aripiprazole Zentiva |
|
INN |
aripiprazole |
|
Marketing-authorisation applicant |
Zentiva, k.s. |
|
Therapeutic indication |
Treatment of schizophrenia and treatment and prevention of manic episodes in bipolar I disorder |
|
More information |
|
Name of medicine |
Duloxetine Mylan |
|
INN |
duloxetine |
|
Marketing-authorisation applicant |
Generics UK Limited |
|
Therapeutic indication |
Treatment of major depressive disorder, diabetic peripheral neuropathic pain and generalised anxiety disorder |
|
More information |
|
Name of medicine |
Pregabalin Mylan |
|
INN |
pregabalin |
|
Marketing-authorisation applicant |
Generics UK Limited |
|
Therapeutic indication |
Treatment of epilepsy and generalised anxiety disorder |
|
More information |
|
Name of medicine |
Pregabalin Mylan Pharma |
|
INN |
pregabalin |
|
Marketing-authorisation applicant |
Generics UK Limited |
|
Therapeutic indication |
Treatment of neuropathic pain, epilepsy and generalised anxiety disorder |
|
More information |
|
Name of medicine |
Pregabalin Sandoz |
|
INN |
pregabalin |
|
Marketing-authorisation applicant |
Sandoz GmbH |
|
Therapeutic indication |
Treatment of neuropathic pain, epilepsy and generalised anxiety disorder |
|
More information |
|
Name of medicine |
Pregabalin Sandoz GmbH |
|
INN |
pregabalin |
|
Marketing-authorisation applicant |
Sandoz GmbH |
|
Therapeutic indication |
Treatment of epilepsy and generalised anxiety disorder |
|
More information |
Positive recommendation on new therapeutic indications
|
Name of medicine |
Esmya |
|
INN |
ulipristal |
|
Marketing-authorisation holder |
Gedeon Richter Plc |
|
More information |
|
Name of medicine |
Invega |
|
INN |
paliperidone |
|
Marketing-authorisation holder |
Janssen-Cilag International N.V. |
|
More information |
|
Name of medicine |
Levemir |
|
INN |
insulin detemir |
|
Marketing-authorisation holder |
Novo Nordisk A/S |
|
More information |
|
Name of medicine |
Relistor |
|
INN |
methylnaltrexone bromide |
|
Marketing-authorisation holder |
TMC Pharma Services Ltd |
|
More information |
|
Name of medicine |
Resolor |
|
INN |
prucalopride |
|
Marketing-authorisation holder |
Shire Pharmaceuticals Ireland Ltd |
|
More information |
|
Name of medicine |
Tygacil |
|
INN |
tigecycline |
|
Marketing-authorisation holder |
Pfizer Limited |
|
More information |
Public health recommendation
|
Name of medicine |
Sovaldi, Daklinza, Harvoni |
|
More information |
EMA recommends avoidance of certain hepatitis C medicines and amiodarone together |
Outcome of arbitration procedure
|
Name of medicine |
Merisone and Myoson |
|
INN |
tolperisone |
|
Marketing-authorisation holder |
Meditop Pharmaceutical Co Ltd |
|
More information |
Withdrawal of application
|
Name of medicine |
Duloxetine Sandoz |
|
INN |
duloxetine |
|
More information |
Other updates
|
Opinions on annual re-assessments, renewals of marketing authorisations and accelerated assessment procedures |
|
Opinions on safety variations |
|
Guidelines and concept papers adopted |
|
Overview of invented names reviewed in March 2015 by the Name Review Group (NRG) |
|
Organisational matters |
|
Opinions on consultation procedures on ancillary medicinal substances in medical devices |
European Medicines Agency, 24.04.2015 (tB).