HAUPTMENÜ
AWARDS
Forschergeist gefragt: 14. Novartis Oppenheim-Förderpreis für MS-Forschung ausgelobt
FernstudiumCheck Award: Deutschlands beliebteste Fernhochschule bleibt die SRH Fernhochschule
Vergabe der Wissenschaftspreise der Deutschen Hochdruckliga und der Deutschen Hypertoniestiftung
Den Patientenwillen auf der Intensivstation im Blick: Dr. Anna-Henrikje Seidlein…
Wissenschaft mit Auszeichnung: Herausragende Nachwuchsforscher auf der Jahrestagung der Deutschen…
VERANSTALTUNGEN
Wichtigster Kongress für Lungen- und Beatmungsmedizin ist erfolgreich gestartet
Virtuelle DGHO-Frühjahrstagungsreihe am 22.03. / 29.03. / 26.04.2023: Herausforderungen in…
Pneumologie-Kongress vom 29. März bis 1. April im Congress Center…
Die Hot Topics der Hirnforschung auf dem DGKN-Kongress für Klinische…
Deutscher Schmerz- und Palliativtag 2023 startet am 14.3.
DOC-CHECK LOGIN
Five new medicines, including one orphan, recommended for approval
Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 23-26 March 2015
London (March 27, 2015) – The CHMP has recommended granting a marketing authorisation for Lenvima (lenvatinib) for the treatment of adults with progressive, locally advanced or metastatic differentiated thyroid carcinoma, whose disease has progressed despite receiving radioactive iodine. Lenvima was reviewed under EMA’s accelerated assessment program. This program provides for an expedited review of medicines that, if approved, would significantly improve the treatment of this serious condition. The medicine also received an orphan designation in 2013 because the condition it is intended to treat was considered a rare disease. For more information on Lenvima, please see the press release in the grid below.
The Committee has recommended granting a marketing authorisation for Gardasil 9, a human papillomavirus (HPV) vaccine, for the prevention of certain diseases caused by nine types of HPV (types 6, 11, 16, 18, 31, 33, 45, 52, 58). Gardasil 9 covers five more HPV types than Gardasil. For more information on Gardasil 9, please see the press release in the grid below.
Akynzeo (netupitant / palonosetron) has been recommended for marketing authorisation for the prevention of chemotherapy-induced nausea and vomiting.
Synjardy (empagliflozin / metformin) received a positive opinion from the Committee for the treatment of type 2 diabetes.
The generic medicine Voriconazole Hospira (voriconazole) also received a positive opinion from the CHMP for the treatment of fungal infections.
One recommendation on extension of therapeutic indication
The Committee recommended an extension of indication for Tamiflu to include the treatment of influenza in infants below one year of age.
Outcome of periodic review
The CHMP has adopted recommendations for Aclasta (zoledronic acid), following a periodic review of the medicine by the PRAC. Aclasta is one of several bisphosphonate medicines with a known risk of osteonecrosis of the jaw. The review concluded that the risk with this medicine remains very low, but has recommended a number of measures to minimise the risk, including an update to the product information and the introduction of a patient reminder card.
Similar measures are planned over the course of 2015/2016 for other intravenous bisphosphonates and another medicine, denosumab, which also has an osteonecrosis risk.
More information is available in the public health communication in the grid below.
Withdrawal of application
The application for a marketing authorisation for Ketoconazole AID-SCFM (ketoconazole) has been withdrawn. A question-and-answer document on this withdrawal is available in the grid below.
Agenda and minutes
The agenda of the March 2015 meeting is published on the EMA website. The minutes of the meeting will be published during the week following the April CHMP meeting.
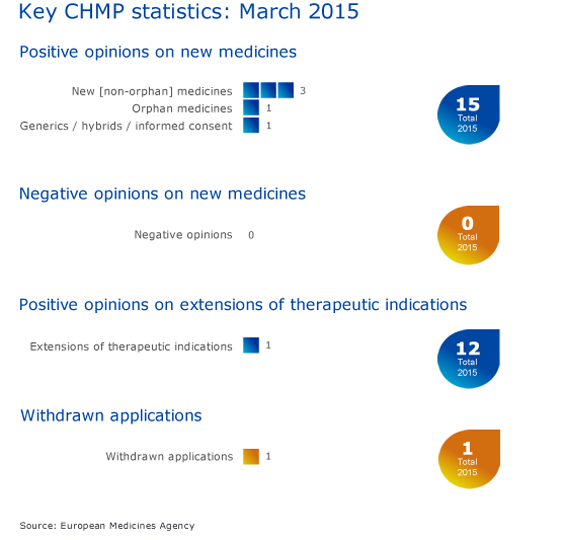
CHMP statistics
Key figures from the March 2015 CHMP meeting are represented in the graphic below.
More information on this, and all other outcomes of the CHMP March 2015 meeting, is available in the grid below.

Positive recommendations on new medicines
|
Name of medicine |
Akynzeo |
|
International non-proprietary name (INN) |
netupitant / palonosetron |
|
Marketing-authorisation applicant |
Helsinn Birex Pharmaceuticals Ltd |
|
Therapeutic indication |
Prevention of chemotherapy-induced nausea and vomiting |
|
More information |
|
Name of medicine |
Gardasil 9 |
|
Common name |
human papillomavirus vaccine [types 6, 11, 16, 18, 31, 33, 45, 52, 58] (recombinant, adsorbed) |
|
Marketing-authorisation applicant |
Sanofi Pasteur MSD SNC |
|
Therapeutic indication |
Treatment of HPV diseases |
|
More information |
Summary of opinion for Gardasil 9 |
|
Name of medicine |
Lenvima |
|
INN |
lenvatinib |
|
Marketing-authorisation applicant |
Eisai Europe Ltd |
|
Therapeutic indication |
Treatment of progressive, locally advanced or metastatic, differentiated thyroid cancer |
|
More information |
Summary of opinion for Lenvima Press release: Accelerated assessment fast-tracks Lenvima to benefit patients with thyroid cancer |
|
Name of medicine |
Synjardy |
|
INN |
empagliflozin / metformin |
|
Marketing-authorisation applicant |
Boehringer Ingelheim GmbH |
|
Therapeutic indication |
Treatment of type 2 diabetes |
|
More information |
Positive recommendation on new generic medicine
|
Name of medicine |
Voriconazole Hospira |
|
INN |
voriconazole |
|
Marketing-authorisation applicant |
Hospira UK Limited |
|
Therapeutic indication |
Treatment of fungal infections |
|
More information |
Positive recommendation on new therapeutic indication
|
Name of medicine |
Tamiflu |
|
INN |
oseltamivir |
|
Marketing-authorisation holder |
Roche Registration Ltd |
|
More information |
Public-health recommendation
|
Name of medicine |
Aclasta |
|
INN |
zoledronic acid |
|
Marketing-authorisation holder |
Novartis Europharm Limited |
|
More information |
Further measures to minimise risk of osteonecrosis of the jaw with bisphosphonate medicine |
Outcome of harmonisation procedure
|
Name of medicine |
Ikorel and Dancor |
|
INN |
nicorandil |
|
Marketing-authorisation holder |
Sanofi-Aventis group of companies and associated companies / Merck group of companies and associated companies |
|
More information |
Withdrawal of application
|
Name of medicine |
Ketoconazole AID-SCFM |
|
INN |
ketoconazole |
|
More information |
Other updates
|
Opinions on annual re-assessments, renewals of marketing authorisations and accelerated assessment procedures |
|
Opinions on safety variations |
|
Guidelines and concept papers adopted |
|
Overview of invented names reviewed in March 2015 by the Name Review Group (NRG) |
|
Organisational matters |
|
Opinions on consultation procedures on ancillary medicinal substances in medical devices |
European Medicines Agency, 27.03.2015 (tB).