HAUPTMENÜ
AWARDS
Forschergeist gefragt: 14. Novartis Oppenheim-Förderpreis für MS-Forschung ausgelobt
FernstudiumCheck Award: Deutschlands beliebteste Fernhochschule bleibt die SRH Fernhochschule
Vergabe der Wissenschaftspreise der Deutschen Hochdruckliga und der Deutschen Hypertoniestiftung
Den Patientenwillen auf der Intensivstation im Blick: Dr. Anna-Henrikje Seidlein…
Wissenschaft mit Auszeichnung: Herausragende Nachwuchsforscher auf der Jahrestagung der Deutschen…
VERANSTALTUNGEN
Wichtigster Kongress für Lungen- und Beatmungsmedizin ist erfolgreich gestartet
Virtuelle DGHO-Frühjahrstagungsreihe am 22.03. / 29.03. / 26.04.2023: Herausforderungen in…
Pneumologie-Kongress vom 29. März bis 1. April im Congress Center…
Die Hot Topics der Hirnforschung auf dem DGKN-Kongress für Klinische…
Deutscher Schmerz- und Palliativtag 2023 startet am 14.3.
DOC-CHECK LOGIN
Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 29 March – 1 April 2016
-
Seven new medicines, including one advanced therapy, recommended for approval
London, UK (April 1, 2016) – The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) recommended seven new medicines, including one advanced therapy medicinal product (ATMP), for approval at its March meeting.
The CHMP recommendedgranting a marketing authorisation for Strimvelis, a new gene therapy intended for the treatment of patients with adenosine-deaminase-deficient severe combined immunodeficiency (ADA-SCID), who have no matching donor for a stem cell transplant. Strimvelis has an orphan designation and is an ATMP. For more information, please see the press release in the grid below.
The Committee recommended granting a conditional marketing authorisation for Darzalex (daratumumab) for the treatment of relapsed and refractory multiple myeloma. Darzalex has an orphan designation and was reviewed under EMA’s accelerated assessment scheme. For more information, please see the press release in the grid below.
The Committee recommended granting a marketing authorisation for Galafold (migalastat) for the treatment of Fabry disease, a rare genetic disorder. Galafold has an orphan designation. For more information, please see the press release in the grid below.
Pandemic influenza vaccine H5N1 MedImmune also received a positive opinion from the CHMP. This is the first pandemic live attenuated influenza vaccine against avian influenza (H5N1) to be recommended for approval in the European Union (EU). The vaccine is intended for pandemic preparedness.
A biosimilarmonoclonal antibody, Flixabi (infliximab), was granted a positive opinion by the Committee for the treatment of rheumatoid arthritis, adult and paediatric Crohn’s disease, ulcerative colitis, paediatric ulcerative colitis, ankylosing spondylitis, psoriatic arthritis and psoriasis.
The CHMP also granted a positive opinion for the informed consent application Neparvis (sacubitril / valsartan) for the treatment of chronic heart failure with reduced ejection fraction. In an informed consent application, reference is made to an authorised medicine and the marketing authorisation holder of the reference medicine has given consent to the use of their dossier in the application procedure. The reference product for Neparvis is Entresto.
A generic medicine, Palonosetron Accord (palonosetron), received a positive opinion from the Committee for the prevention of nausea and vomiting associated with chemotherapy.
Three recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for Halaven, Humira and Opdivo.
Re-examination procedure concluded
The CHMP concluded a re-examination procedure, issuing a final negative opinion for Dropcys (mercaptamine). A questions-and-answers document on this opinion is available below.
Review on vancomycin
The CHMP started a review of medicines containing the antibiotic vancomycin as part of its efforts to update product information of older antibacterial agents in the context of the fight against antimicrobial resistance. The revision of product information for critically important antibiotics is considered an important way of promoting appropriate use to ensure they remain available to EU patients. For more information, please see the start of referral documents in the grid below.
Review on Symbioflor 2
The Committee started a review of the medicine Symbioflor 2 (Escherichia coli bacteria), which is authorised in some Member States of the EU for treating diseases affecting the stomach and gut including irritable bowel syndrome.For more information, please see the start of referral documents in the grid below.
Review of medicines for which studies have been conducted at Alkem BE, India
The CHMP also started a review of medicinesfor which studies have been conducted at the Alkem BE site in Mumbai, India. This follows a good clinical practice (GCP) inspection of this site which raised concerns regarding study data used to support the marketing authorisation applications of some medicines in the EU. For more information, please see the start of referral documents in the grid below.
Agenda and minutes
The agenda of the March 2016 meeting is published on EMA’s website. Minutes of the February 2016 CHMP meeting will be published next week.
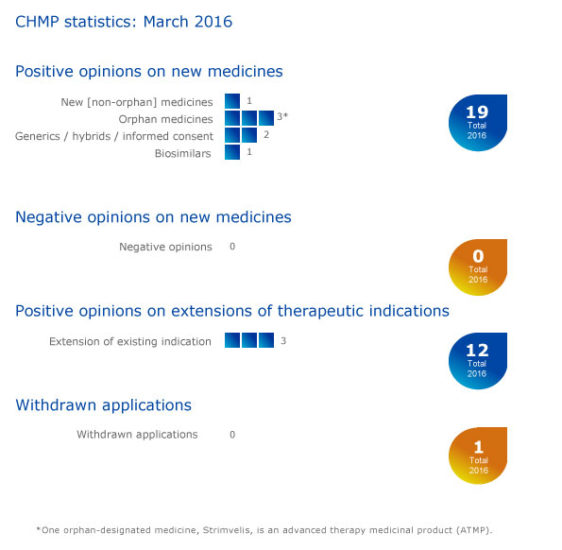
CHMP statistics
Key figures from the March 2016 CHMP meeting are represented in the graphic below.
More information on this, and all other outcomes of the CHMP’s March 2016 meeting, is available in the grid below.

Positive recommendations on new medicines
|
Name of medicine |
Darzalex |
|
International non-proprietary name (INN) |
daratumumab |
|
Marketing-authorisation applicant |
Janssen-Cilag International N.V. |
|
Therapeutic indication |
Treatment of relapsed and refractory multiple myeloma |
|
More information |
Summary of opinion for Darzalex Press release: New treatment for patients with multiple myeloma |
|
Name of medicine |
Galafold |
|
INN |
migalastat |
|
Marketing-authorisation applicant |
Amicus Therapeutics UK Ltd |
|
Therapeutic indication |
Treatment of Fabry disease |
|
More information |
Summary of opinion for Galafold Press release: First oral treatment for Fabry disease recommended for approval in the EU |
|
Name of medicine |
Pandemic influenza vaccine H5N1 MedImmune |
|
INN |
pandemic influenza vaccine (H5N1) (live attenuated, nasal) |
|
Marketing-authorisation applicant |
MedImmune LLC |
|
Therapeutic indication |
Prophylaxis of influenza |
|
More information |
Summary of opinion for pandemic influenza vaccine H5N1 MedImmune |
|
Name of medicine |
Strimvelis |
|
INN |
autologous cd34+ enriched cell fraction that contains cd34+ cells transduced with retroviral vector that encodes for the human ada cdna sequence |
|
Marketing-authorisation applicant |
GlaxoSmithKline Trading Services |
|
Therapeutic indication |
Treatment of severe combined immunodeficiency due to adenosine deaminase deficiency (ADA-SCID) |
|
More information |
Summary of opinion for Strimvelis Press release: New gene therapy for the treatment of children with ultra-rare immune disorder recommended for approval |
Positive recommendation on new informed-consent application
|
Name of medicine |
Neparvis |
|
INN |
sacubitril / valsartan |
|
Marketing-authorisation applicant |
Novartis Europharm Ltd |
|
Therapeutic indication |
Treatment of chronic heart failure with reduced ejection fraction |
|
More information |
Positive recommendation on new generic medicine
|
Name of medicine |
Palonosetron Accord |
|
International non-proprietary name |
palonosetron |
|
Marketing-authorisation applicant |
Accord Healthcare Ltd |
|
Therapeutic indication |
Prevention of nausea and vomiting associated with cancer chemotherapy |
|
More information |
Positive recommendation on new biosimilar medicine
|
Name of medicine |
Flixabi |
|
INN |
infliximab |
|
Marketing-authorisation applicant |
Samsung Bioepis UK Limited |
|
Therapeutic indication |
Treatment of rheumatoid arthritis, adult and paediatric Crohn’s disease, ulcerative colitis, paediatric ulcerative colitis, ankylosing spondylitis, psoriatic arthritis and psoriasis |
|
More information |
Re-examination of recommendation for new medicine
|
Name of medicine |
Dropcys |
|
International non-proprietary name (INN) |
mercaptamine |
|
Marketing-authorisation applicant |
Lucane Pharma |
|
Therapeutic indication |
Treatment of corneal cystine deposits |
|
More information |
Questions and answers on refusal of marketing authorisation for Dropcys |
Positive recommendations on extensions of therapeutic indications
|
Name of medicine |
Halaven |
|
INN |
eribulin |
|
Marketing-authorisation holder |
Eisai Europe Ltd |
|
More information |
|
Name of medicine |
Humira |
|
INN |
adalimumab |
|
Marketing-authorisation holder |
AbbVie Ltd |
|
More information |
|
Name of medicine |
Opdivo |
|
INN |
nivolumab |
|
Marketing-authorisation holder |
Bristol-Myers Squibb Pharma EEIG |
|
More information |
Other procedures
|
Name of medicine |
Trevicta (previously known as Paliperidone Janssen) |
|
INN |
paliperidone |
|
Marketing-authorisation holder |
Janssen-Cilag International NV |
|
More information |
Summary of opinion for Trevicta (previously known as Paliperidone Janssen) |
Starts of referrals
|
Name of medicine |
Alkem – Article-31 |
|
Marketing-authorisation holder |
Alkem Laboratories Ltd (India) |
|
More information |
|
Name of medicine |
Symbioflor 2 – Article-31 |
|
INN |
Escherichia coli bacteria (cells and autolysate) |
|
More information |
|
Name of medicine |
Vancomycin-containing medicines – Article-31 |
|
INN |
vancomycin |
|
More information |
Other updates
|
Opinions on annual re-assessments, renewals of marketing authorisations and accelerated assessment procedures |
|
Opinions on safety variations |
|
Guidelines and concept papers adopted |
|
Organisational matters |
|
Opinions on consultation procedures on ancillary medicinal substances in medical devices |
European Medicines Agency (EMA), 01.04.2016 (tB).