HAUPTMENÜ
AWARDS
Forschergeist gefragt: 14. Novartis Oppenheim-Förderpreis für MS-Forschung ausgelobt
FernstudiumCheck Award: Deutschlands beliebteste Fernhochschule bleibt die SRH Fernhochschule
Vergabe der Wissenschaftspreise der Deutschen Hochdruckliga und der Deutschen Hypertoniestiftung
Den Patientenwillen auf der Intensivstation im Blick: Dr. Anna-Henrikje Seidlein…
Wissenschaft mit Auszeichnung: Herausragende Nachwuchsforscher auf der Jahrestagung der Deutschen…
VERANSTALTUNGEN
Wichtigster Kongress für Lungen- und Beatmungsmedizin ist erfolgreich gestartet
Virtuelle DGHO-Frühjahrstagungsreihe am 22.03. / 29.03. / 26.04.2023: Herausforderungen in…
Pneumologie-Kongress vom 29. März bis 1. April im Congress Center…
Die Hot Topics der Hirnforschung auf dem DGKN-Kongress für Klinische…
Deutscher Schmerz- und Palliativtag 2023 startet am 14.3.
DOC-CHECK LOGIN
Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 12-15 September 2016
- Eleven medicines recommended for approval, including three medicines for cancer
London, UK (September 16, 2016) – The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) recommended eleven medicines for approval at its September meeting. The CHMP recommended granting a conditional marketing authorisation for Lartruvo (olaratumab) for the treatment of adults with advanced soft tissue sarcoma. Lartruvo is to be used in combination with doxorubicin (a chemotherapy medicine) in patients with advanced soft tissue sarcoma for whom surgery or radiotherapy is not suitable, and who have not been previously treated with doxorubicin. Lartruvo was reviewed under EMA’s accelerated assessment mechanism and has an orphan designation. For more information, please see the press release in the grid below.
The Committee recommended granting a marketing authorisation for Ibrance (palbociclib) for the treatment of women with locally-advanced or metastatic breast cancer. Ibrance is to be used to treat breast cancer that is hormone receptor (HR)-positive and human epidermal growth factor receptor 2 (HER2)–negative. For more information, please see the press release in the grid below.
The CHMP recommended granting a conditional marketing authorisation for Ninlaro (ixazomib) for the treatment of multiple myeloma. This follows a re-examination of the Committee’s earlier negative opinion. Ninlaro is a new medicine that is taken orally to treat multiple myeloma, and it has an orphan designation. Please see the questions-and-answers document in the grid below for more information on this opinion.
Glyxambi (empagliflozin / linagliptin) was recommended for approval for the treatment of type 2 diabetes.
Parsabiv (etelcalcetide) was recommended for approval for the treatment of secondary hyperparathyroidism.
The Committee recommended granting a marketing authorisation for the orphan medicine SomaKit-TOC (edotreotide) for the diagnosis of gastro-entero-pancreatic neuroendrocrine tumours.
One hybrid application, Chenodeoxycholic acid sigma-tau (chenodeoxycholic acid), received a positive opinion from the CHMP for the treatment of cerebrotendinous xanthomatosis. Hybrid applications rely in part on the results of pre-clinical tests and clinical trials for a reference product and in part on new data. This medicine has an orphan designation.
Four generic medicines were recommended for approval by the Committee: Ivabradine JensenR (ivabradine) and Ivabradine Zentiva (ivabradine) to treat angina pectoris and heart failure, Emtricitabine / Tenofovir disoproxil Zentiva (emtricitabine / tenofovir disoproxil) to treat HIV infection and Granpidam (sildenafil) for the treatment of patients with pulmonary arterial hypertension.
Two recommendations on extensions of therapeutic indications
The Committee recommended extensions of indications for NovoRapid and Stelara.
Outcome of review of compliance with good manufacturing practice (GMP) at Pharmaceutics International
The CHMP recommended that medicines manufactured by Pharmaceutics International Inc., located in the United States, should no longer be available in the European Union, except Ammonaps (sodium phenylbutyrate), which is used to treat a rare disease and is considered critical for public health. The recommendation is the outcome of a review into issues with GMP at Pharmaceutics International Inc. For more information, please see the public health communication in the grid below.
Withdrawals of applications
The application for a marketing authorisation for Cokiera (dasabuvir / ombitasvir / paritaprevir / ritonavir) has been withdrawn. Cokiera was intended for the treatment of chronic hepatitis C. A questions-and-answers document on this withdrawal is available below.
A request to extend the indication of Adempas (riociguat) to the treatment of pulmonary arterial hypertension has also been withdrawn. A questions-and-answers document on this withdrawal is available below.
Agenda and minutes
The agenda of the September 2016 meeting is published on EMA’s website. Minutes of the July 2016 CHMP meeting will be published next week.
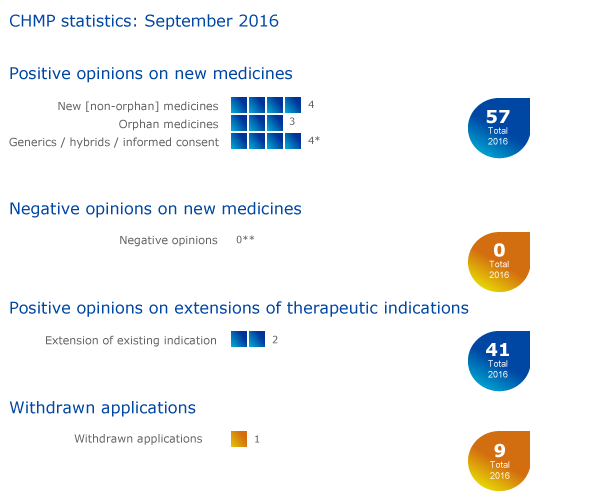
CHMP statistics
Key figures from the September 2016 CHMP meeting are represented in the graphic below.
More information on this, and all other outcomes of the CHMP’s September 2016 meeting, is available in the grid below.

- * Mysildecard (sildenafil), a generic of Revatio, received a positive opinion from the CHMP by written procedure in July 2016. This is reflected in the total number of positive opinions on new medicines for 2016.
- ** Ninlaro received a positive opinion at the September CHMP following a re-examination. Therefore the number of negative opinions for 2016 has been revised to 0.
Positive recommendations on new medicines
|
Name of medicine |
Glyxambi |
|
International non-proprietary name (INN) |
empagliflozin / linagliptin |
|
Marketing-authorisation applicant |
Boehringer Ingelheim International GmbH |
|
Therapeutic indication |
Treatment of type 2 diabetes mellitus |
|
More information |
|
Name of medicine |
Ibrance |
|
INN |
palbociclib |
|
Marketing-authorisation applicant |
Pfizer Limited |
|
Therapeutic indication |
Treatment of locally advanced or metastatic breast cancer |
|
More information |
Summary of opinion for Ibrance Press release: New treatment for breast cancer |
|
Name of medicine |
Lartruvo |
|
INN |
olaratumab |
|
Marketing-authorisation applicant |
Eli Lilly Nederland B.V. |
|
Therapeutic indication |
Treatment of soft tissue sarcoma |
|
More information |
Summary of opinion for Lartruvo Press release: New treatment for patients with soft tissue sarcoma
|
|
Name of medicine |
Ninlaro |
|
INN |
ixazomib |
|
Marketing-authorisation applicant |
Takeda Pharma A/S |
|
Therapeutic indication |
Treatment of multiple myeloma |
|
More information |
Summary of opinion for Ninlaro Questions and answers on the positive opinion on the marketing authorisation for Ninlaro |
|
Name of medicine |
Parsabiv |
|
INN |
etelcalcetide |
|
Marketing-authorisation applicant |
Amgen Europe B.V. |
|
Therapeutic indication |
Treatment of secondary hyperparathyroidism |
|
More information |
|
Name of medicine |
SomaKit TOC |
|
INN |
edotreotide |
|
Marketing-authorisation applicant |
Advanced Accelerator Applications |
|
Therapeutic indication |
Diagnosis of gastro-entero-pancreatic neuroendocrine tumours |
|
More information |
Positive recommendations on new generic medicines
|
Name of medicine |
Emtricitabine/Tenofovir disoproxil Zentiva |
|
INN |
emtricitabine / tenofovir disoproxil |
|
Marketing-authorisation applicant |
Zentiva k.s. |
|
Therapeutic indication |
Treatment of HIV infection |
|
More information |
Summary of opinion for Emtricitabine/Tenofovir disoproxil Zentiva |
|
Name of medicine |
Granpidam |
|
INN |
sildenafil |
|
Marketing-authorisation applicant |
Accord Healthcare Ltd |
|
Therapeutic indication |
Treatment of pulmonary arterial hypertension |
|
More information |
|
Name of medicine |
Ivabradine JensonR |
|
INN |
ivabradine |
|
Marketing-authorisation applicant |
JensonR+Limited |
|
Therapeutic indication |
Treatment of angina pectoris and heart failure |
|
More information |
|
Name of medicine |
Ivabradine Zentiva |
|
INN |
ivabradine |
|
Marketing-authorisation applicant |
Zentiva, k.s. |
|
Therapeutic indication |
Treatment of angina pectoris and heart failure |
|
More information |
Positive recommendation on a new hybrid medicine
|
Name of medicine |
Chenodeoxycholic acid sigma-tau |
|
INN |
chenodeoxycholic acid |
|
Marketing-authorisation applicant |
Sigma-tau Arzneimittel GmbH |
|
Therapeutic indication |
Treatment of cerebrotendinous xanthomatosis |
|
More information |
Positive recommendations on extensions of therapeutic indications
|
Name of medicine |
NovoRapid |
|
INN |
insulin aspart |
|
Marketing-authorisation holder |
Novo Nordisk A/S |
|
More information |
|
Name of medicine |
Stelara |
|
INN |
ustekinumab |
|
Marketing-authorisation holder |
Janssen-Cilag International NV |
|
More information |
Public-health recommendation
|
Name of the procedure |
Pharmaceutics International Inc |
|
More information |
Outcome of harmonisation procedure
|
Name of medicine |
Clenil and associated names |
|
INN |
Beclometasone dipropionate |
|
Marketing-authorisation holder |
Chiesi and associated companies |
|
More information |
Withdrawals of application
|
Name of medicine |
Adempas |
|
INN |
riociguat |
|
More information |
Questions and answers on the withdrawal of the marketing authorisation application for Adempas |
|
Name of medicine |
Cokiera |
|
INN |
dasabuvir / ombitasvir / paritaprevir / ritonavir |
|
More information |
Questions and answers on the withdrawal of the marketing authorisation application for Cokiera |
Other opinion
|
Name of medicine |
Abilify |
|
INN |
aripiprazole |
|
More information |
Other updates
|
|
|
|
|
European Medicines Agency (EMA), 16.06.2016 (tB).